Identification
- Summary
Human immunoglobulin G is a purified form of human immunoglobulin G and other proteins used to treat immunodeficiency and a wide variety of autoimmune disorders.
- Brand Names
- Alyglo, Asceniv, Bivigam, Cuvitru, Flebogamma, Gamastan, Gammagard, Gammaked, Gammaplex, Gamunex, Hizentra, Hyqvia 5 G / 50 Ml Kit, Igivnex, Kiovig, Octagam, Panzyga, Privigen, Xembify
- Generic Name
- Human immunoglobulin G
- DrugBank Accession Number
- DB00028
- Background
Intravenous immunoglobulin (IVIg) is a mixture of IgG1 and other antibodies derived from healthy human plasma via Cohn fractionation. The purification process includes cold alcohol fractionation, polyethylene glycol precipitation, and ion exchange chromatography. IVIg contains the same distribution of IgG antibody subclasses as is found in the general human population. IgG subclasses are fully represented in the following proportions: 70.3% IgG1, 24.7% IgG2, 3.1% IgG3, and 1.9% IgG4. IVIg is used in the treatment of immunodeficiencies, as well as autoimmune and inflammatory disorders.
- Type
- Biotech
- Groups
- Approved, Investigational
- Biologic Classification
- Protein Based Therapies
Blood factors - Protein Structure
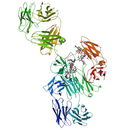
- Protein Chemical Formula
- C6332H9826N1692O1980S42
- Protein Average Weight
- 142682.3 Da
- Sequences
>IGG1 PSALTQPPSASGSLGQSVTISCTGTSSDVGGYNYVSWYQQHAGKAPKVIIYEVNKRPSGV PDRFSGSKSGNTASLTVSGLQAEDEADYYCSSYEGSDNFVFGTGTKVTVLGQPKANPTVT LFPPSSEELQANKATEVCLISDFYPGAVTVAWKADGSPVKAGVETTKPSKQSNNKYAASS YLSLTPEQWKSHRSYSCQVTHEGSTVEKTVAPTECSPLVLQESGPGLVKPSEALSLTCTV SGDSINTILYYWSWIRQPPGKGLEWIGYIYYSGSTYGNPSLKSRVTISVNTSKNQFYSKL SSVTAADTAVYYCARVPLVVNPWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGC LVKDYFPQPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNH KPSNTKVDKRVAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPQVKFNWYV DGVQVHNAKTKPREQQYNSTYRVVSVLTVLHQNWLDGKEYKCKVSNKALPAPIEKTISKA KGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLD SDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSL
>IgA2 ELVMTQSPSSLSASVGDRVNIACRASQGISSALAWYQQKPGKAPRLLIYDASNLESGVPS RFSGSGSGTDFTLTISSLQPEDFAIYYCQQFNSYPLTFGGGTKVEIKRTVAAPSVFIFPP SDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLT LSKADYEKHKVYACEVTHQGLSSPVTKSFNRGECQVKLLEQSGAEVKKPGASVKVSCKAS GYSFTSYGLHWVRQAPGQRLEWMGWISAGTGNTKYSQKFRGRVTFTRDTSATTAYMGLSS LRPEDTAVYYCARDPYGGGKSEFDYWGQGTLVTVSSASPTSPKVFPLSLDSTPQDGNVVV ACLVQGFFPQEPLSVTWSESGQNVTARNFPPSQDASGDLYTTSSQLTLPATQCPDGKSVT CHVKHYTNPSQDVTVPCPVPPPPPCCHPRLSLHRPALEDLLLGSEANLTCTLTGLRDASG ATFTWTPSSGKSAVQGPPERDLCGCYSVSSVLPGCAQPWNHGETFTCTAAHPELKTPLTA NITKSGNTFRPEVHLLPPPSEELALNELVTLTCLARGFSPKDVLVRWLQGSQELPREKYL TWASRQEPSQGTTTFAVTSILRVAAEDWKKGDTFSCMVGHEALPLAFTQKTIDRLAGKPT HVNVSVVMAEVDGTCY
Download FASTA Format- Synonyms
- Aerosolized pooled immune globulin
- Human gammaglobulin
- Human IGG
- Human immunoglobulin G
- Human normal immunoglobulin
- Immune globulin (human)
- Immune globulin human
- Immunoglobulin (human)
- Immunoglobulin G (human)
- Immunoglobulin G, human
- Intravenous immune globulin (human)
- Intravenous immunoglobulin (IVIg)
Pharmacology
- Indication
Human immunoglobulin G is indicated for the following conditions:
Primary Immunodeficiency
- for the treatment of primary immunodeficiency in adult and pediatric patients5,6,7,8,9,10,11,12,13,15,16,17,18,20
- in combination with hyaluronidase (human recombinant) for the treatment of primary immunodeficiency in patients ≥2 years of age.21
Immune Thrombocytopenic Purpura (ITP)
- for the treatment of acute or chronic immune thrombocytopenic purpura in adult and pediatric patients8,10,12,13,14,15,16
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- for the treatment of CIDP in adult patients11,13,15,16,18
- in combination with hyaluronidase (human recombinant) as a maintenance therapy in adults with chronic inflammatory demyelinating polyneuropathy (CIDP)19
Multifocal Motor Neuropathy (MMN)
- for maintenance therapy to improve muscle strength and disability in adult patients with MMN11
Prophylaxis of Bacterial Infection
- for the prevention of bacterial infections in patients with hypogammaglobulinemia and/or B-cell chronic lymphocytic leukemia12
Coronary Artery Aneurysm Associated With Kawasaki Syndrome
- for the prevention of coronary artery aneurysms in pediatric patients with Kawasaki syndrome12
Dermatomyositis
- for the treatment of dermatomyositis in adult patients14
 Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.
Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.- Associated Conditions
Indication Type Indication Combined Product Details Approval Level Age Group Patient Characteristics Dose Form Treatment of Acute idiopathic thrombocytopenic purpura •••••••••••• ••••••••• Prevention of Bacterial infections •••••••••••• ••• Prevention of Bacterial infections •••••••••••• ••• Treatment of Chronic idiopathic thrombocytopenic purpura •••••••••••• ••••••••••• ••••• •••••• Treatment of Chronic idiopathic thrombocytopenic purpura •••••••••••• •••••• ••••••••• •••••••••• ••••••••• ••• - Associated Therapies
- Contraindications & Blackbox Warnings
 Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API
Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API- Pharmacodynamics
Used as a replacement therapy in inherited humoral immunodeficiency disorders such as severe combined immunodeficiency syndrome, x-linked agammaglobulinemia, and Wiskott-Aldrich Syndrome. The immunoglobulins target, bind and kill bacterial cells as well as viral particles. IgG is the monomeric immunoglobulin of which there are four subclasses (IgG1, IgG2, IgG3 and IgG4) in differing abundances (66%, 23%, 7% and 4%). IgAs represent about 15% of the immunoglobulins in the blood. These target inhaled or ingested pathogens.
- Mechanism of action
IVIg interacts with a number of different components of the immune system, including cytokines, complement, Fc receptors and several cell surface immunocompetent molecules. IVIg also impacts different effector cells of the immune system (B and T lymphocytes, dendritic cells, etc.) and regulates a wide range of genes. Its main mechanism of actions are believed to be Fc-dependent and F(ab')2-dependent. IVIg competitively blocks gamma Fc receptors, preventing the binding and ingestion of phagocytes and suppressing platelet depletion. IVIg contains a number of different antobodies, which prevent infection by attaching to the surface of invading pathogens and aiding in their disposal before they can infect cells. Antibodies remove pathogens via complement activation, agglutination or precipitation, pathogen receptor blocking, macrophage “tagging” or neutralization (via binding) of pathogen toxins. Intact IVIg and F(ab′)2 fragments of IVIg can also neutralize the activity of various autoantibodies. By triggering the production of interleukin-1 receptor antagonist, IVIg modulates of the production of cytokines and cytokine antagonists. It also prevents the generation of the C5b-9 membrane attack complex and subsequent complement-mediated tissue damage by binding active complement components.
Target Actions Organism AHigh affinity immunoglobulin gamma Fc receptor I antagonistHumans AHigh affinity immunoglobulin gamma Fc receptor IB antagonistHumans ALow affinity immunoglobulin gamma Fc region receptor II-a antagonistHumans ALow affinity immunoglobulin gamma Fc region receptor II-b antagonistHumans ALow affinity immunoglobulin gamma Fc region receptor II-c antagonistHumans ALow affinity immunoglobulin gamma Fc region receptor III-A antagonistHumans ALow affinity immunoglobulin gamma Fc region receptor III-B antagonistHumans AComplement C3 binderHumans AComplement C4-A binderHumans AComplement C4-B binderHumans AComplement C5 binderHumans - Absorption
Not Available
- Volume of distribution
Not Available
- Protein binding
Not Available
- Metabolism
- Not Available
- Route of elimination
Not Available
- Half-life
>20 hours (mammalian reticulocytes, in vitro).
- Clearance
Not Available
- Adverse Effects
 Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.
Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.- Toxicity
Not Available
- Pathways
- Not Available
- Pharmacogenomic Effects/ADRs
- Not Available
Interactions
- Drug Interactions
- This information should not be interpreted without the help of a healthcare provider. If you believe you are experiencing an interaction, contact a healthcare provider immediately. The absence of an interaction does not necessarily mean no interactions exist.
Drug Interaction Integrate drug-drug
interactions in your softwareAbciximab The risk or severity of adverse effects can be increased when Human immunoglobulin G is combined with Abciximab. Adalimumab The risk or severity of adverse effects can be increased when Human immunoglobulin G is combined with Adalimumab. Aducanumab The risk or severity of adverse effects can be increased when Human immunoglobulin G is combined with Aducanumab. Alemtuzumab The risk or severity of adverse effects can be increased when Human immunoglobulin G is combined with Alemtuzumab. Alirocumab The risk or severity of adverse effects can be increased when Human immunoglobulin G is combined with Alirocumab. - Food Interactions
- No interactions found.
Products
 Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.
Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.- International/Other Brands
- ClairYg
- Brand Name Prescription Products
Name Dosage Strength Route Labeller Marketing Start Marketing End Region Image Alyglo Liquid 100 mg/1mL Intravenous GC Biopharma Corp. 2023-12-15 Not applicable US Asceniv Liquid 5 g/50mL Intravenous Adma Biologics, Inc. 2019-04-01 Not applicable US Bivigam Injection, solution 5 g/50mL Intravenous Adma Biologics, Inc. 2013-01-21 Not applicable US Bivigam Injection, solution 10 g/100mL Intravenous ADMA Biologics, Inc 2013-02-04 2021-09-30 US Bivigam Injection, solution 1 g/10mL Intravenous Kedrion Biopharma, Inc. 2013-02-04 Not applicable US - Over the Counter Products
Name Dosage Strength Route Labeller Marketing Start Marketing End Region Image Iypoly Aerosol, spray 0.15 g/100mL Nasal Whoniz 2021-12-23 Not applicable US - Mixture Products
Name Ingredients Dosage Route Labeller Marketing Start Marketing End Region Image Hyqvia Human immunoglobulin G (20 g / 200 mL) + Hyaluronidase (human recombinant) (1600 unit / 10 mL) Solution Subcutaneous Takeda 2023-11-07 Not applicable Canada Hyqvia Human immunoglobulin G (2.5 g / 25 mL) + Hyaluronidase (human recombinant) (200 unit / 1.25 mL) Solution Subcutaneous Takeda 2023-11-07 Not applicable Canada Hyqvia Human immunoglobulin G (100 mg/1mL) + Hyaluronidase (human recombinant) (160 [USP'U]/1mL) Kit; Solution Subcutaneous Takeda Pharmaceuticals America, Inc. 2014-09-12 Not applicable US Hyqvia Human immunoglobulin G (100 mg/1mL) + Hyaluronidase (human recombinant) (160 [USP'U]/1mL) Kit; Solution Subcutaneous Takeda Pharmaceuticals America, Inc. 2014-09-12 Not applicable US Hyqvia Human immunoglobulin G (10 g / 100 mL) + Hyaluronidase (human recombinant) (800 unit / 5 mL) Solution Subcutaneous Takeda 2023-11-07 Not applicable Canada - Unapproved/Other Products
Name Ingredients Dosage Route Labeller Marketing Start Marketing End Region Image Iypoly Human immunoglobulin G (0.15 g/100mL) Aerosol, spray Nasal Whoniz 2021-12-23 Not applicable US PENTAGLOBIN 50 ML FLAKON, 1 ADET Human immunoglobulin G (50 mg/ml) Injection Intravenous KANSUK LABORATUVARI SAN. VE TİC. A.Ş. 2013-01-29 Not applicable Turkey
Categories
- Drug Categories
- Chemical TaxonomyProvided by Classyfire
- Description
- Not Available
- Kingdom
- Organic Compounds
- Super Class
- Organic Acids
- Class
- Carboxylic Acids and Derivatives
- Sub Class
- Amino Acids, Peptides, and Analogues
- Direct Parent
- Peptides
- Alternative Parents
- Not Available
- Substituents
- Not Available
- Molecular Framework
- Not Available
- External Descriptors
- Not Available
- Affected organisms
- Humans and other mammals
- Bacteria and protozoa
- Various viruses
Chemical Identifiers
- UNII
- 66Y330CJHS
- CAS number
- 308067-58-5
References
- Synthesis Reference
Wolfgang Stephan, "Production of intravenously applicable native human immune globulin having a normal half-life." U.S. Patent US4082734, issued July, 1970.
US4082734- General References
- Bayry J, Fournier EM, Maddur MS, Vani J, Wootla B, Siberil S, Dimitrov JD, Lacroix-Desmazes S, Berdah M, Crabol Y, Oksenhendler E, Levy Y, Mouthon L, Sautes-Fridman C, Hermine O, Kaveri SV: Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. J Autoimmun. 2011 Feb;36(1):9-15. doi: 10.1016/j.jaut.2010.09.006. Epub 2010 Dec 9. [Article]
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Stangel M, Pul R: Basic principles of intravenous immunoglobulin (IVIg) treatment. J Neurol. 2006 Sep;253 Suppl 5:V18-24. [Article]
- Emmi L, Chiarini F: The role of intravenous immunoglobulin therapy in autoimmune and inflammatory disorders. Neurol Sci. 2002 Apr;23 Suppl 1:S1-8. [Article]
- FDA Approved Drug Products: Cutaquig (human immunoglobulin G-hipp) solution for subcutaneous infusion [Link]
- FDA Approved Drug Products: Asceniv (human immunoglobulin G-sira) for intravenous injection [Link]
- FDA Approved Drug Products: Bivigam (human immunoglobulin G) for intravenous injection [Link]
- FDA Approved Drug Products: Carimune NF (human immunoglobulin G) for intravenous injection [Link]
- FDA Approved Drug Products: Flebogamma 5% (human immunoglobulin G) solution for intravenous administration [Link]
- FDA Approved Drug Products: Flebogamma 10% (human immunoglobulin G) for intravenous administration [Link]
- FDA Approved Drug Products: Gammagard Liquid (human immunoglobulin G) for subcutaneous and intravenous administration [Link]
- FDA Approved Drug Products: Gammagard S/D (human immunoglobulin G, solvent detergent treated) for intravenous administration [Link]
- FDA Approved Drug Products: Gamunex-C (human immunoglobulin G, purified) for intravenous injection [Link]
- FDA Approved Drug Products: Octagam (human immunoglobulin G) solution for intravenous administration [Link]
- FDA Approved Drug Products: Panzyga (human immunoglobulin G-ifas) liquid for intravenous administration [Link]
- FDA Approved Drug Products: Privigen (human immunoglobulin G) liquid for intravenous administration [Link]
- FDA Approved Drug Products: Cuvitru (human immunoglobulin G) solution for subcutaneous injection [Link]
- FDA Approved Drug Products: Hizentra (human immunoglobulin G) liquid for subcutaneous injection [Link]
- FDA Approved Drug Products: Hyqvia (human immunoglobulin G/human recombinant hyaluronidase) solution for subcutaneous injection [Link]
- FDA Approved Drug Products: Xembify (human immunoglobulin G-klhw) solution for subcutaneous injection [Link]
- FDA Approved Drug Products: Hyqvia (human immunoglobulin G/human recombinant hyaluronidase) solution for subcutaneous injection [Link]
- External Links
- UniProt
- P01877
- Genbank
- J00221
- PubChem Substance
- 46508774
- 1426680
- PharmGKB
- PA164754884
- RxList
- RxList Drug Page
- Drugs.com
- Drugs.com Drug Page
Clinical Trials
- Clinical Trials
Phase Status Purpose Conditions Count 4 Active Not Recruiting Treatment Chronic immune thrombocytopenia 1 4 Completed Other Humoral Immune Responses 1 4 Completed Prevention Anti-Hepatitis A Antibody Levels in Heathy Subjects 1 4 Completed Prevention Common Variable Immune Deficiency (CVID) / Hyper-IgM Syndrome / Primary Immune Deficiency Disorders (PIDD) / Wiskott-Aldrich Syndrome (WAS) / X-Linked Agammaglobulinemia 1 4 Completed Prevention IgG Deficiency / Infection 1
Pharmacoeconomics
- Manufacturers
- Not Available
- Packagers
- Alpha Therapeutic Corp.
- American Red Cross
- Baxter International Inc.
- Biotest Pharmaceuticals
- Cangene Corp.
- CSL Behring LLC
- Grifols SA
- Massachusetts Public Health
- Massbiologics
- Medimmune Inc.
- Novartis AG
- Octapharma USA
- Talecris Biotherapeutics
- Dosage Forms
Form Route Strength Liquid Intravenous 100 mg/1mL Liquid Intravenous 5 g/50mL Injection, solution Intramuscular; Subcutaneous Solution Intramuscular; Subcutaneous 800 mg Solution Intramuscular; Subcutaneous 320 mg Injection, solution Intravenous 1 g/10mL Injection, solution Intravenous 10 g/100mL Injection, solution Intravenous 5 g/50mL Solution Intravenous 50 mg Injection, powder, lyophilized, for solution Intravenous 12 g/1 Injection, powder, lyophilized, for solution Intravenous 3 g/1 Injection, powder, lyophilized, for solution Intravenous 6 g/1 Solution Subcutaneous 1 g / 6 mL Solution Subcutaneous 1.65 g / 10 mL Solution Subcutaneous 165 mg/1mL Solution Subcutaneous 2 g / 12 mL Solution Subcutaneous 3.3 g / 20 mL Solution Subcutaneous 4 g / 24 mL Solution Subcutaneous 8 g / 48 mL Injection, solution Parenteral Injection, solution Subcutaneous 200 mg/1mL Injection, solution Subcutaneous Solution Subcutaneous 200 mg Solution Parenteral Injection, solution Intravenous 0.5 g/10mL Injection Intravenous 5 % Solution, concentrate Intravenous 20 g Solution Intravenous 5 % Solution, concentrate Intravenous 0.5 g Solution, concentrate Intravenous 10 g Solution Intravenous 2.5 g Solution Intravenous 20 g Injection, solution Intravenous 0.05 g/1mL Injection, solution Intravenous 50 MG/ML Solution Intravenous 100 mg/ml Injection, solution Intramuscular 0.165 g/1mL Liquid Intramuscular 16.5 % Injection 1.65 g Solution Intramuscular 18 % Solution Intravenous 50 mg / mL Powder, for solution Intravenous 50 MG/ML Injection, solution Intravenous; Subcutaneous 100 mg/1mL Injection, powder, lyophilized, for solution; kit Intravenous 50 mg/1mL Kit Intravenous 50 mg/1mL Kit; powder, for solution; solution Intravenous 10 g / vial Kit; powder, for solution; solution Intravenous 5 g / vial Powder, for solution Intravenous 2.5 g / vial Powder, for solution Intravenous 0.5 g / vial Injection, powder, for solution Intravenous 52 mg/ml Injection, powder, for solution Intravenous Injection Intravenous; Subcutaneous 10 g/100mL Solution Subcutaneous 165 mg Solution Intravenous 5 g/50mL Solution Intravenous 5 g/100mL Liquid Intramuscular 165 mg / mL Injection, solution Intravascular 50 mg/1ml Injection Intravenous 1 g/1g Solution Intravenous; Subcutaneous 10 g / 100 mL Injection Intravenous 0.1 g/ml Injection, solution Intravenous 10 g Injection, solution Intravenous 20 g Injection, solution Intravenous 5 g Injection, solution Intravenous; Subcutaneous Injection, solution Intravenous; Subcutaneous 0.1 g/mL Injection, solution Intravenous 5 g/100ml Injection, solution Subcutaneous 200 MG/ML Liquid Subcutaneous 0.2 g/1mL Solution Subcutaneous 200 mg / mL Solution Subcutaneous 4 g Injection, solution Subcutaneous 200 g/L Solution Subcutaneous 1 g Solution Subcutaneous 2 g Injection, solution Subcutaneous 100 MG/ML Kit; solution Subcutaneous Solution Subcutaneous Injection 100 mg/mL Solution Subcutaneous 100 mg Solution Intravenous Injection, solution Intravenous 2.5 G Solution Intravenous 1 g Solution Intravenous 100000 g Solution Intravenous 10 gr/200ml Injection, solution Intravenous 1 g/20ml Solution Intravenous 5000000 mg Injection, solution Intravenous 2.5 g/50ml Solution Intravenous 5 gr/100ml Solution Intravenous 50 mg/ml Solution Intravenous 50 mg (titer) Liquid Intravenous 50 mg / mL Solution Intravenous 10 g / 100 mL Solution Intravenous 5 g / 100 mL Solution Parenteral 160 mg Solution, concentrate Intravenous 50 mg Injection Intravenous 6 g/100mL Injection, solution Intravenous 100 G/L Injection, solution Intravenous 50 G/L Injection, solution Intravenous 10 g/200ml Injection, solution Intravenous 20 g/200ml Injection, solution Intravascular 100 MG/ML Injection, solution Intravenous 100 MG/ML Kit; liquid; powder, for solution Intravenous Aerosol, spray Nasal Aerosol, spray Nasal 0.15 g/100mL Injection, solution Parenteral 160 MG/ML Injection, solution Intravenous Solution Intravenous; Subcutaneous 100 mg Injection, solution Intravenous; Subcutaneous 10 gr/100ml Injection, solution Intravenous; Subcutaneous 2.5 gr/25ml Injection, solution Intravenous 20 gr/200ml Injection, solution Intravenous 30 gr/300ml Injection, solution Intravenous; Subcutaneous 5 gr/50ml Injection, solution Subcutaneous 160 MG/ML Injection, solution Intravenous 5 % Solution Intravenous 100 mg / mL Solution Intravenous 100 mg Solution Intravenous 50 mg/1mL Solution Intravenous 1000 mg Injection, solution Intramuscular; Subcutaneous 165 MG/ML Injection, powder, for solution Parenteral 70 mg/ml Solution Intravenous 100 mg/1mL Injection Intramuscular 160 mg/ml Solution Intravascular 50 mg/1ml Injection Intravenous 50 mg/ml Liquid Intravenous 10 g/100mL Liquid Intravenous 20 g/200mL Liquid Intravenous 40 g/400mL Solution Intravenous 10 % Injection Parenteral 10 g/100ml Solution Intravenous 10 g Solution Intravenous 5 g Injection Parenteral Injection Parenteral 5 g/50ml Solution Intravenous 100 g/L Injection, solution Subcutaneous 300 mg/300mg Solution Intravenous 120 g / L Injection, solution Intramuscular; Subcutaneous 160 MG/ML Injection, powder, for solution Intravenous 2.5 g Injection, powder, lyophilized, for solution Intravenous 10 g/200ml Injection, powder, lyophilized, for solution Intravenous 5 g/100ml Injection, powder, lyophilized, for solution Intravenous 5 g Injection, solution Intravenous 50 mg/1ml Injection, solution 160 MG/ML Solution Subcutaneous 160 mg / mL Solution Subcutaneous 160 mg/1mL Liquid Intramuscular; Intravenous 1500 [iU]/1mL Liquid Intramuscular; Intravenous 15000 [iU]/1mL Liquid Intramuscular; Intravenous 2500 [iU]/1mL Liquid Intramuscular; Intravenous 5000 [iU]/1mL Liquid Intramuscular; Intravenous 600 [iU]/1mL Solution Subcutaneous 20 % Solution Subcutaneous 200 mg/1mL Injection, solution Intravascular 20 g/100ml - Prices
Unit description Cost Unit Hypertet s-d 250 unit syringe 324.23USD syringe Hyperrab s-d vial 224.07USD ml Nabi-hb vial 176.34USD ml Hyperhep b s-d vial 165.04USD ml Hizentra 1 gram/5 ml vial 30.24USD ml Hizentra 2 gram/10 ml vial 30.24USD ml Hizentra 4 gram/20 ml vial 30.24USD ml Gamastan s-d vial 24.44USD ml Gammagard liquid 10% vial 13.79USD ml Gamunex 10% vial 11.6USD ml Immune globulin vial 9.34USD ml Flebogamma 5% vial 4.84USD ml Flebogamma dif 5% vial 4.84USD ml DrugBank does not sell nor buy drugs. Pricing information is supplied for informational purposes only.- Patents
- Not Available
Properties
- State
- Liquid
- Experimental Properties
Property Value Source melting point (°C) 61 °C (FAB fragment), 71 °C (whole mAb) Vermeer, A.W.P. & Norde, W., Biophys. J. 78:394-404 (2000) hydrophobicity -0.331 Not Available isoelectric point 8.13 Not Available
Targets

- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antagonist
- General Function
- Receptor signaling protein activity
- Specific Function
- High affinity receptor for the Fc region of immunoglobulins gamma. Functions in both innate and adaptive immune responses.
- Gene Name
- FCGR1A
- Uniprot ID
- P12314
- Uniprot Name
- High affinity immunoglobulin gamma Fc receptor I
- Molecular Weight
- 42631.525 Da
References
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antagonist
- General Function
- Immunoglobulin receptor activity
- Specific Function
- May bind to the Fc region of immunoglobulins gamma with a low affinity compared to FCGR1A. May function in the humoral immune response.
- Gene Name
- FCGR1B
- Uniprot ID
- Q92637
- Uniprot Name
- High affinity immunoglobulin gamma Fc receptor IB
- Molecular Weight
- 32231.795 Da
References
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antagonist
- General Function
- Not Available
- Specific Function
- Binds to the Fc region of immunoglobulins gamma. Low affinity receptor. By binding to IgG it initiates cellular responses against pathogens and soluble antigens. Promotes phagocytosis of opsonized ...
- Gene Name
- FCGR2A
- Uniprot ID
- P12318
- Uniprot Name
- Low affinity immunoglobulin gamma Fc region receptor II-a
- Molecular Weight
- 35000.42 Da
References
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antagonist
- General Function
- Not Available
- Specific Function
- Receptor for the Fc region of complexed or aggregated immunoglobulins gamma. Low affinity receptor. Involved in a variety of effector and regulatory functions such as phagocytosis of immune complex...
- Gene Name
- FCGR2B
- Uniprot ID
- P31994
- Uniprot Name
- Low affinity immunoglobulin gamma Fc region receptor II-b
- Molecular Weight
- 34043.355 Da
References
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antagonist
- General Function
- Transmembrane signaling receptor activity
- Specific Function
- Receptor for the Fc region of complexed immunoglobulins gamma. Low affinity receptor. Involved in a variety of effector and regulatory functions such as phagocytosis of immune complexes and modulat...
- Gene Name
- FCGR2C
- Uniprot ID
- P31995
- Uniprot Name
- Low affinity immunoglobulin gamma Fc region receptor II-c
- Molecular Weight
- 35577.96 Da
References
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antagonist
- General Function
- Not Available
- Specific Function
- Receptor for the Fc region of IgG. Binds complexed or aggregated IgG and also monomeric IgG. Mediates antibody-dependent cellular cytotoxicity (ADCC) and other antibody-dependent responses, such as...
- Gene Name
- FCGR3A
- Uniprot ID
- P08637
- Uniprot Name
- Low affinity immunoglobulin gamma Fc region receptor III-A
- Molecular Weight
- 29088.895 Da
References
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antagonist
- General Function
- Not Available
- Specific Function
- Receptor for the Fc region of immunoglobulins gamma. Low affinity receptor. Binds complexed or aggregated IgG and also monomeric IgG. Contrary to III-A, is not capable to mediate antibody-dependent...
- Gene Name
- FCGR3B
- Uniprot ID
- O75015
- Uniprot Name
- Low affinity immunoglobulin gamma Fc region receptor III-B
- Molecular Weight
- 26215.64 Da
References
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Binder
- General Function
- Receptor binding
- Specific Function
- C3 plays a central role in the activation of the complement system. Its processing by C3 convertase is the central reaction in both classical and alternative complement pathways. After activation C...
- Gene Name
- C3
- Uniprot ID
- P01024
- Uniprot Name
- Complement C3
- Molecular Weight
- 187146.73 Da
References
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Binder
- General Function
- Endopeptidase inhibitor activity
- Specific Function
- Non-enzymatic component of C3 and C5 convertases and thus essential for the propagation of the classical complement pathway. Covalently binds to immunoglobulins and immune complexes and enhances th...
- Gene Name
- C4A
- Uniprot ID
- P0C0L4
- Uniprot Name
- Complement C4-A
- Molecular Weight
- 192783.805 Da
References
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Binder
- General Function
- Non-enzymatic component of the C3 and C5 convertases and thus essential for the propagation of the classical complement pathway. Covalently binds to immunoglobulins and immune complexes and enhances the solubilization of immune aggregates and the clearance of IC through CR1 on erythrocytes. C4A isotype is responsible for effective binding to form amide bonds with immune aggregates or protein antigens, while C4B isotype catalyzes the transacylation of the thioester carbonyl group to form ester bonds with carbohydrate antigens.
- Specific Function
- Carbohydrate binding
- Gene Name
- C4B
- Uniprot ID
- P0C0L5
- Uniprot Name
- Complement C4-B
- Molecular Weight
- 192749.785 Da
References
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Binder
- General Function
- Receptor binding
- Specific Function
- Activation of C5 by a C5 convertase initiates the spontaneous assembly of the late complement components, C5-C9, into the membrane attack complex. C5b has a transient binding site for C6. The C5b-C...
- Gene Name
- C5
- Uniprot ID
- P01031
- Uniprot Name
- Complement C5
- Molecular Weight
- 188303.705 Da
References
- Baerenwaldt A, Biburger M, Nimmerjahn F: Mechanisms of action of intravenous immunoglobulins. Expert Rev Clin Immunol. 2010 May;6(3):425-34. doi: 10.1586/eci.10.9. [Article]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV: Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007 May;27(3):233-45. Epub 2007 Mar 11. [Article]
- Siberil S, Elluru S, Graff-Dubois S, Negi VS, Delignat S, Mouthon L, Lacroix-Desmazes S, Kazatchkine MD, Bayry J, Kaveri SV: Intravenous immunoglobulins in autoimmune and inflammatory diseases: a mechanistic perspective. Ann N Y Acad Sci. 2007 Sep;1110:497-506. [Article]
Drug created at June 13, 2005 13:24 / Updated at February 20, 2024 23:55

